
David Ricks, CEO, Eli Lilly.
Scott Mlyn | CNBC
Eli Lilly CEO David Ricks on Tuesday said Medicare price negotiations, which aim to cut costs for older Americans, could potentially harm drug development.
“I’m really worried about the harm this will do to new cures and possibilities in medicine,” Ricks said in an interview on CNBC’s “The Exchange.”
Ricks was referring to a provision in the Biden administration’s Inflation Reduction Act that will allow the Medicare program to negotiate prices on the costliest prescription drugs each year.
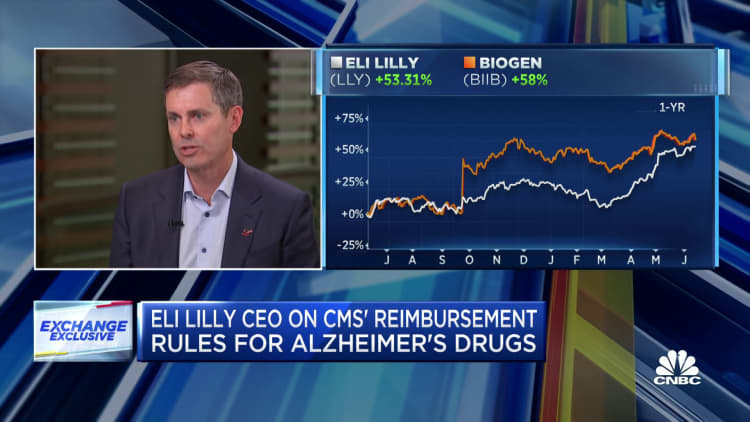
He’s the latest pharmaceutical executive to publicly blast the provision and law at large, which will likely reduce company profits. Global drugmaker Merck last week sued the Biden administration over Medicare price negotiations in a bid to weaken the program.
Ricks said the “biggest problem” with the provision stems from a difference in timeline for negotiating prices on small-molecule drugs — meaning drugs made of chemicals that have low molecular weight — versus biologic medicines, or those derived from living sources such as animals or humans.
Under the Inflation Reduction Act provision, Medicare can start negotiating prices on small-molecule drugs as early as nine years after they receive U.S. Food and Drug Administration approval, compared with 13 years for biologics.
Ricks said that distinction is “going to really truncate investment” in small-molecule drugs, which are “one of the most efficient parts of health care.”
“We’ll get fewer of [small-molecule drugs] because investors are already saying to me, ‘Why would you invest in more small molecules when biologics get 13 years before negotiations?'” Ricks said.
Small-molecule drugs make up 90% of pharmaceutical drugs, according to a study in ScienceDirect.
Novartis CEO Vas Narasimhan in February expressed similar concerns about the varying timeline, saying it’s a top priority of the industry to correct the four-year gap between the two types of drugs, according to Fierce Pharma.
Another provision of the Inflation Reduction Act requires pharmaceutical companies to refund Medicare through rebates if the prices of their drugs rise faster than the rate of inflation.
The first set of eligible prescription drugs was subject to Medicare inflation rebates starting April 1, according to the U.S. Department of Health and Human Services.









